New Tool Uses Ultrasound ‘Tornado’ to Break Down Blood Clots
January 23, 2023
By Matt Shipman, Research Communication Lead, North Carolina State University
Researchers at the Georgia Institute of Technology and North Carolina State University have developed a new tool and technique that uses “vortex ultrasound” – a sort of ultrasonic tornado – to break down blood clots in the brain. The new approach worked more quickly than existing techniques to eliminate clots formed in an in vitro model of cerebral venous sinus thrombosis (CVST).
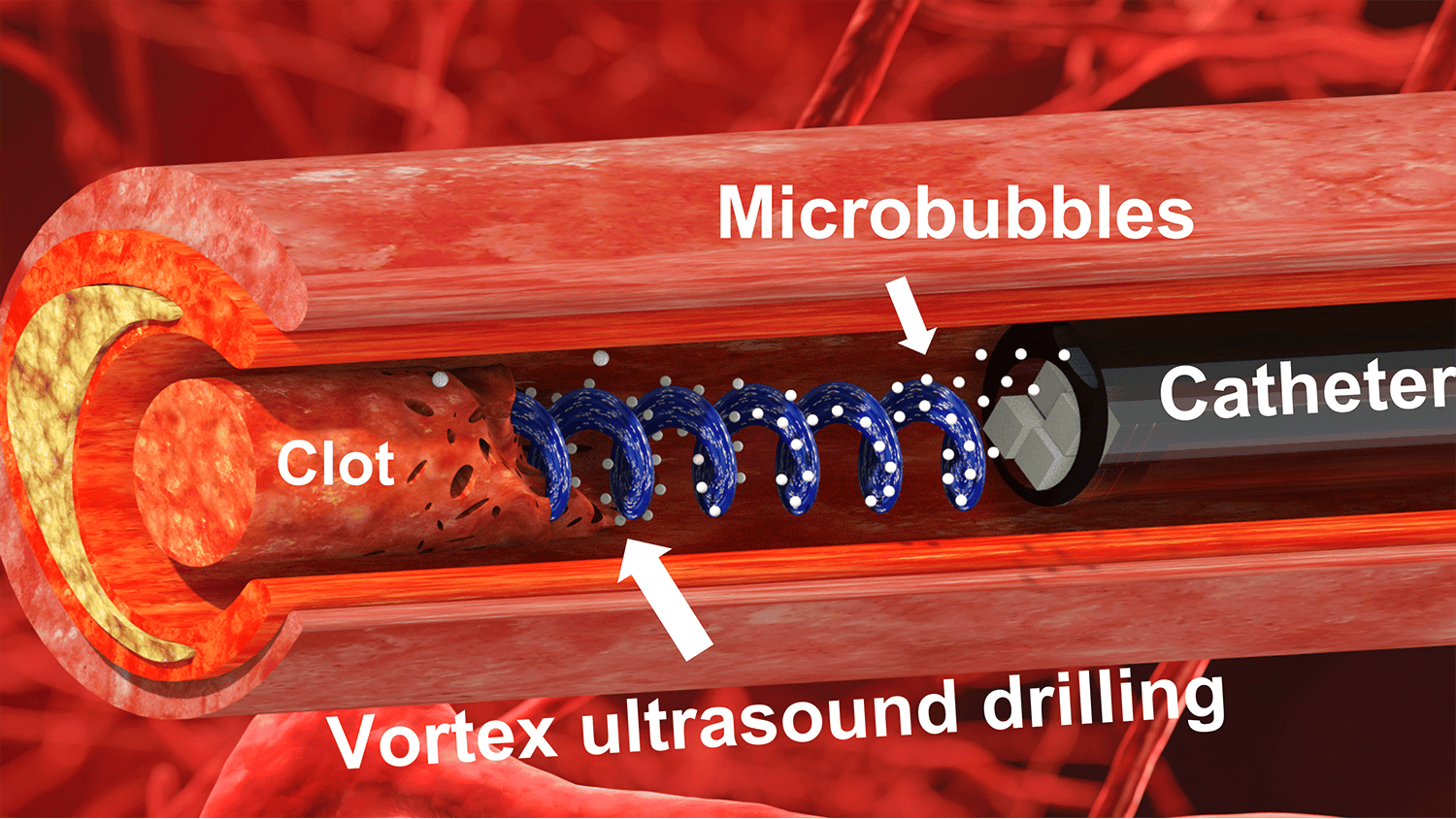
“Our previous work looked at various techniques that use ultrasound to eliminate blood clots using what are essentially forward-facing waves,” says Xiaoning Jiang, co-corresponding author of a paper on the work. “Our new work uses vortex ultrasound, where the ultrasound waves have a helical wavefront.
“In other words, the ultrasound is swirling as it moves forward,” says Jiang, who is the Dean F. Duncan Professor of Mechanical and Aerospace Engineering at North Carolina State University. “Based on our in vitro testing, this approach eliminates blood clots more quickly than existing techniques, largely because of the shear stress induced by the vortex wave.”
“The fact that our new technique works quickly is important, because CVST clots increase pressure on blood vessels in the brain,” says Chengzhi Shi, co-corresponding author of the work and Assistant Professor in the George W. Woodruff School of Mechanical Engineering at Georgia Tech. “This increases the risk of a hemorrhage in the brain, which can be catastrophic for patients."
“Existing techniques rely in large part on interventions that dissolve the blood clot. But this is a time-consuming process. Our approach has the potential to address these clots more quickly, reducing risk for patients.”
CVST occurs when a blood clot forms in the veins responsible for draining blood from the brain. Incidence rates of CVST were between 2 and 3 per 100,000 in the United States in 2018 and 2019, and the incidence rate appears to be increasing.
“Another reason our work here is important is that current treatments for CVST fail in 20-40% of cases,” Jiang says.
The new tool consists of a single transducer that is specifically designed to produce the swirling, vortex effect. The transducer is small enough to be incorporated into a catheter, which is then fed through the circulatory system to the site of the blood clot.
For proof-of-concept in vitro testing, the researchers used cow blood in a 3D-printed model of the cerebral venous sinus.
“Based on available data, pharmaceutical interventions to dissolve CVST blood clots take at least 15 hours, and average around 29 hours,” Shi says. “During in vitro testing, we were able to dissolve an acute blood clot in well under half an hour.”
During any catheterization or surgical intervention there is a potential risk of harm, such as damaging the blood vessel itself. To address this issue, the researchers performed experiments applying vortex ultrasound to animal blood vein samples. Those tests found no damage to the walls of the blood vessels.
The researchers also conducted tests to determine whether the vortex ultrasound caused significant damage to red blood cells. They found that there was not substantial damage to red blood cells.
“The next step is for us to perform tests using an animal model to better establish the viability of this technique for CVST treatment,” Jiang says. “If those tests are successful, we hope to pursue clinical trials.”
“And if the vortex ultrasound ever becomes a clinical application, it would likely be comparable in cost to other interventions used to treat CVST,” says Shi.
The paper, “A Model of High-Speed Endovascular Sonothrombolysis with Vortex Ultrasound-Induced Shear Stress to Treat Cerebral Venous Sinus Thrombosis,” is published in the open-access journal Research, which is a Science Partner Journal. Co-lead authors of the paper are Bohua Zhang, a Ph.D. student at NC State; Huaiyu Wu, a postdoctoral researcher at NC State; and Howuk Kim, a former Ph.D. student at NC State who is now on faculty at Inha University. The paper was co-authored by: Phoebe Welch at Georgia Tech; Ashley Cornett, Greyson Stocker, Gabe Owens and Zhen Xu at the University of Michigan; Raul Nogueira at the University of Pittsburgh Medical Center; Jinwook Kim, a research assistant professor in the Joint Department of Biomedical Engineering at North Carolina State University and the University of North Carolina, Chapel Hill; and Paul Dayton, department chair and William R. Kenan Distinguished Professor in the Joint Department of Biomedical Engineering.
The work was done with support from the National Institutes of Health under grants R01HL141967, R41HL154735, and R21EB027304; and the National Science Foundation, under grant number CMMI-2142555.
